
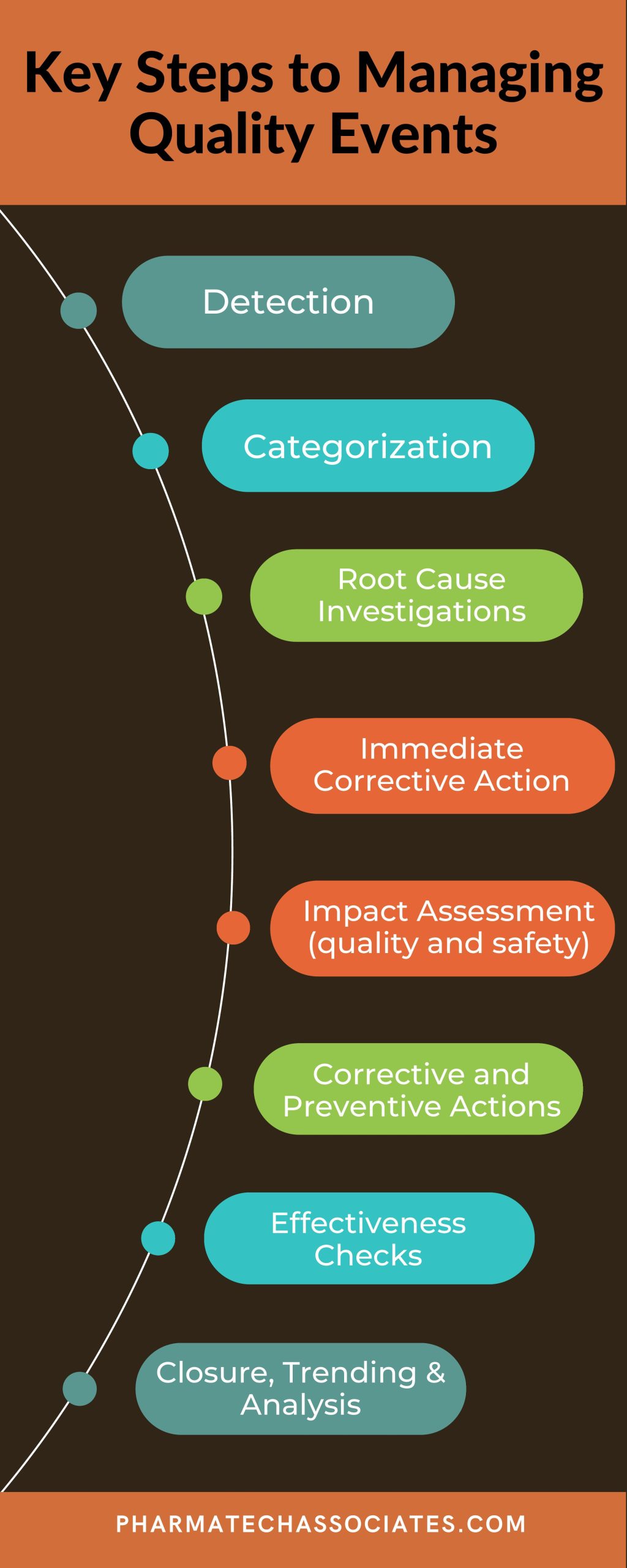
Strong quality systems are required to maintain a successful business and avoid 483’s from FDA inspections. A common cause for citations in industry come from the management of quality events. Here are the basic elements in any quality event management process:
If you need help establishing a quality events management system, Pharmatech Associates can help! Contact us today to learn more.
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541