
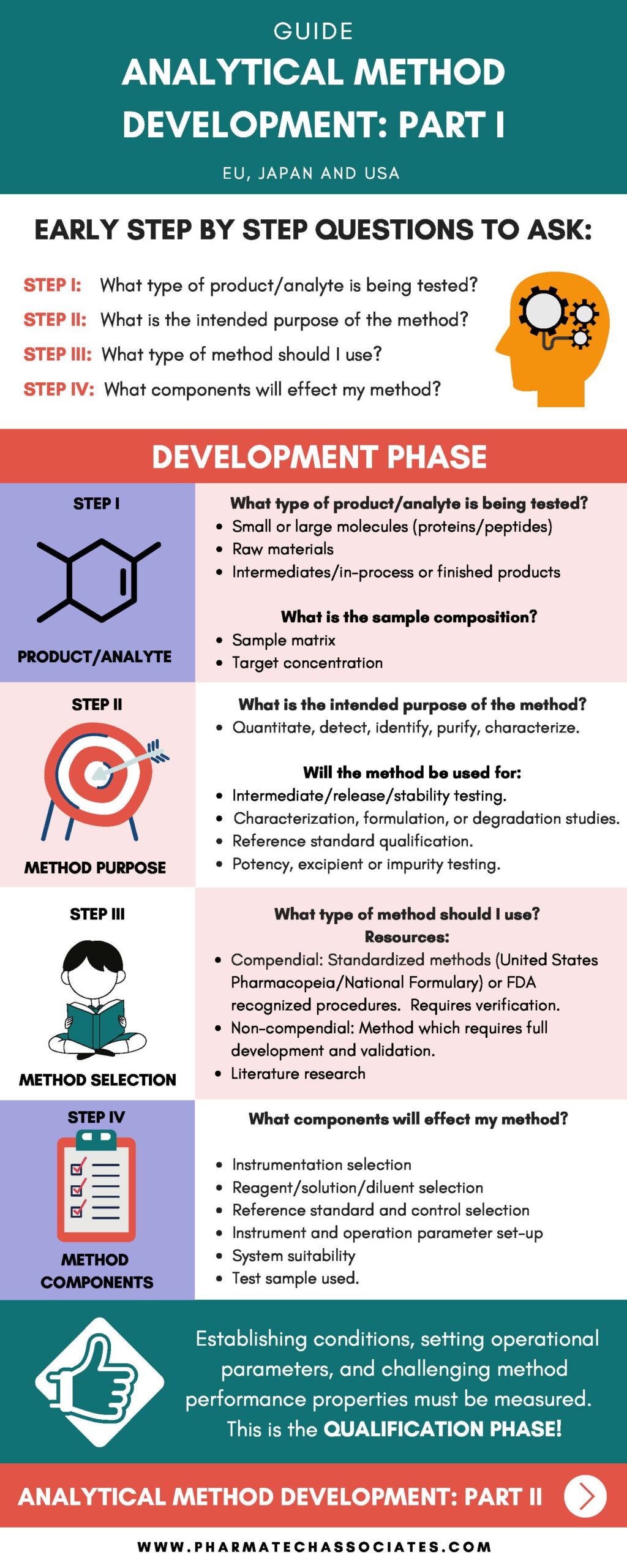
What type of product/analyte is being tested?
What is the sample composition?
What is the intended purpose of the method?
Will the method be used for:
What type of method should I use?
What components will effect my method?
Establishing conditions, setting operations parameters, and challenging method performance properties must be measured. This is the QUALIFICATION PHASE!
It can be tough to define each step of the development stage of your drug, but the experts at Pharmatech Associates are here to help. Contact us today!
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541