
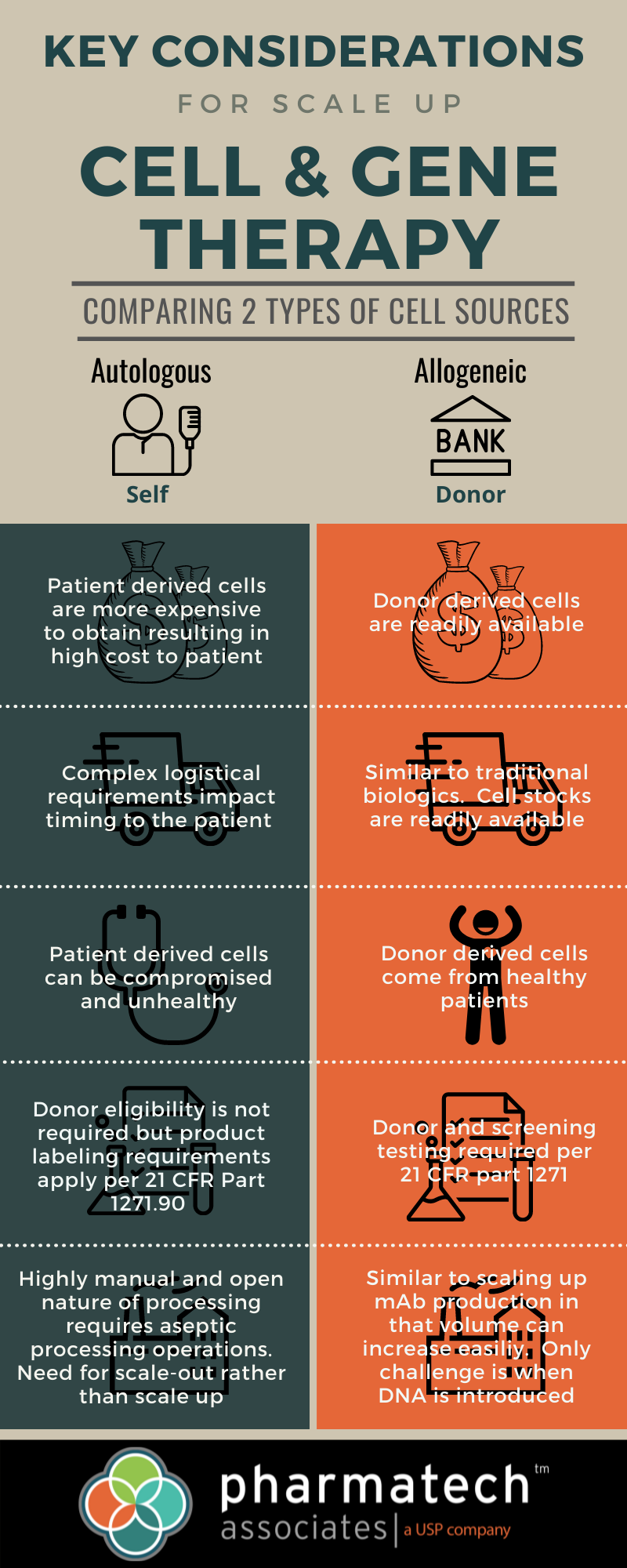
Cost: Patient-derived cells are more expensive to obtain, resulting in high cost to patient.
Logistics: Complex logistical requirements impact timing to the patient.
Cell Condition: Patient-derived cells can be compromised and unhealthy.
Eligibility: Donor eligibility is not required but product labeling requirements apply per CFR Part 1271.90
Scaling: Highly manual and open nature of processing requires aseptic processing operations. Need for scale-out rather than scale up.
Cost: Donor derived sales are readily available.
Logistics: Similar to traditional biologics. Cell stocks are readily available.
Cell Condition: Donor derived cells come from healthy patients.
Eligibility: Donor and screening testing required per 21 CFR part 1271.
Scaling: Similar to scaling up mAb production in that volume can increase easily. Only challenge is when DNA is introduced.
The experts at Pharmatech Associates can help you scale up your cell and/or gene therapy company. Contact us today!
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541