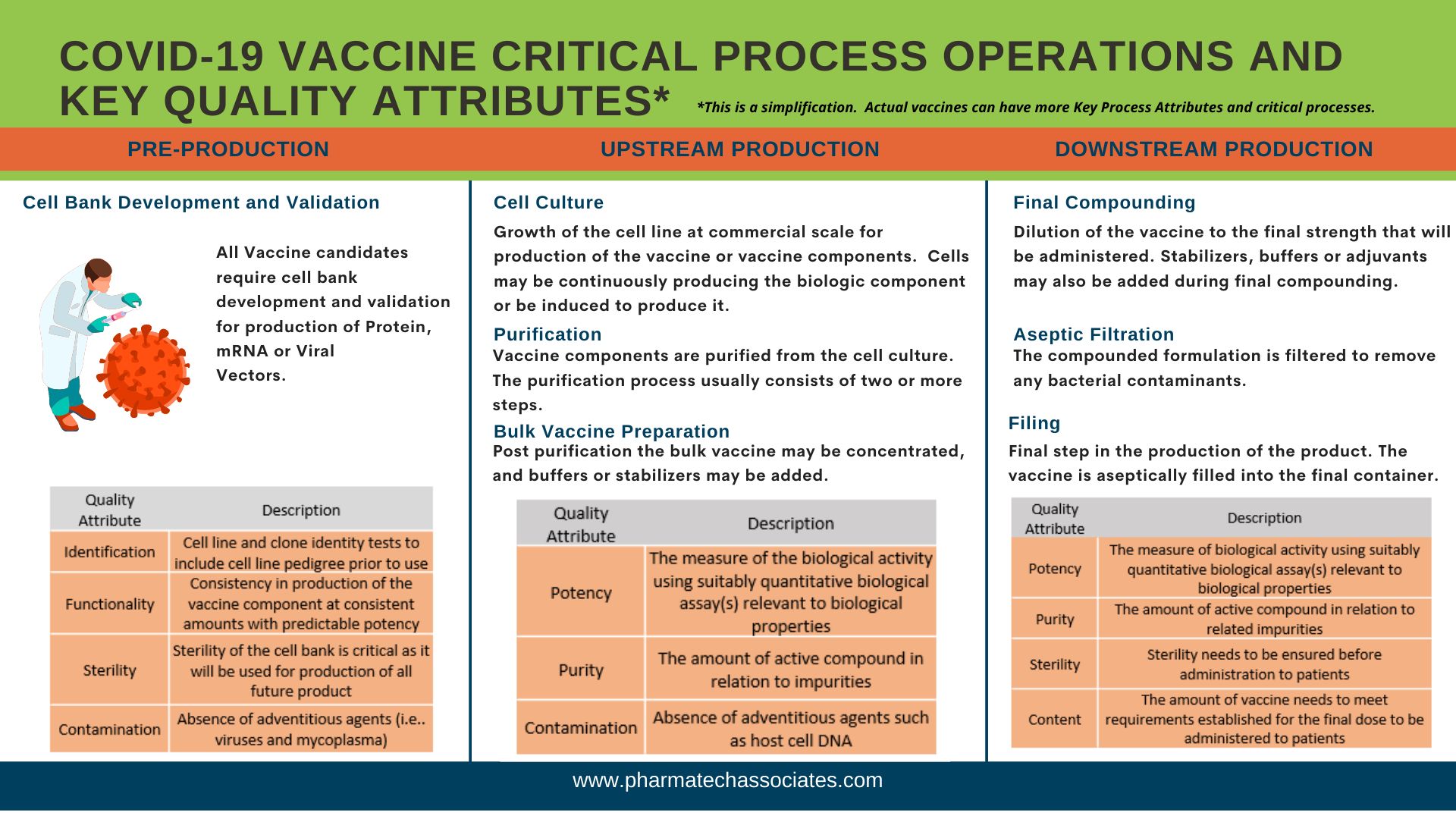
All vaccine candidates require cell bank development and validation for production of protein, mRNA, or viral vectors.
Growth of the cell line at commercial scale for production of the vaccine or vaccine components. Cells may be continuously producing the biologic component or be induced to produce it.
Vaccine components are purified from the cell culture. The purification process usually consists of two or more steps.
Post purification, the bulk vaccine may be concentrated and buffers or stabilizers may be added.
Dilution of the vaccine to the final strength that will be administered. Stabilizers, buffers, or adjuvants may also be added during final compounding.
The compounded formulation is filtered to remove any bacterial contaminants.
Final step in the production of the product. The vaccine is aseptically filled into the final container.
Need help determining your drug development process? Contact us so that we help your company determine the development process of your drug, biologic or medical device.
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541