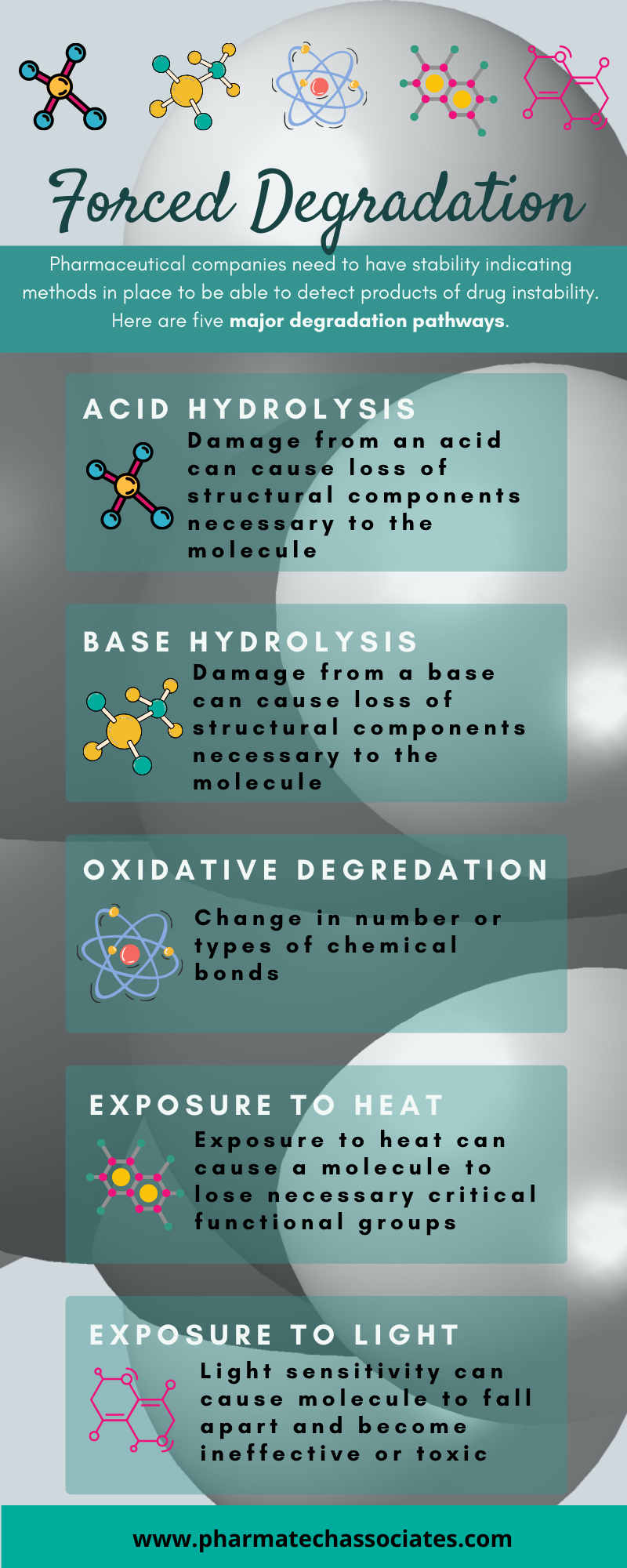
Pharmaceutical companies need to have stability indicating methods in place to be able to detect products of drug instability.
Here are five major degradation pathways:
Damage from an acid can cause loss of structural components necessary to the molecule
Damage from a base can cause loss of structural components necessary to the molecule
Change in number or types of chemical bonds
Exposure to heat can cause a molecule to lose necessary critical functional groups
Light sensitivity can cause molecule to fall apart and become ineffective or toxic
Contact us today to help determine what stability indicating methods you should be using for your drug, biologic, or medical device.
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541