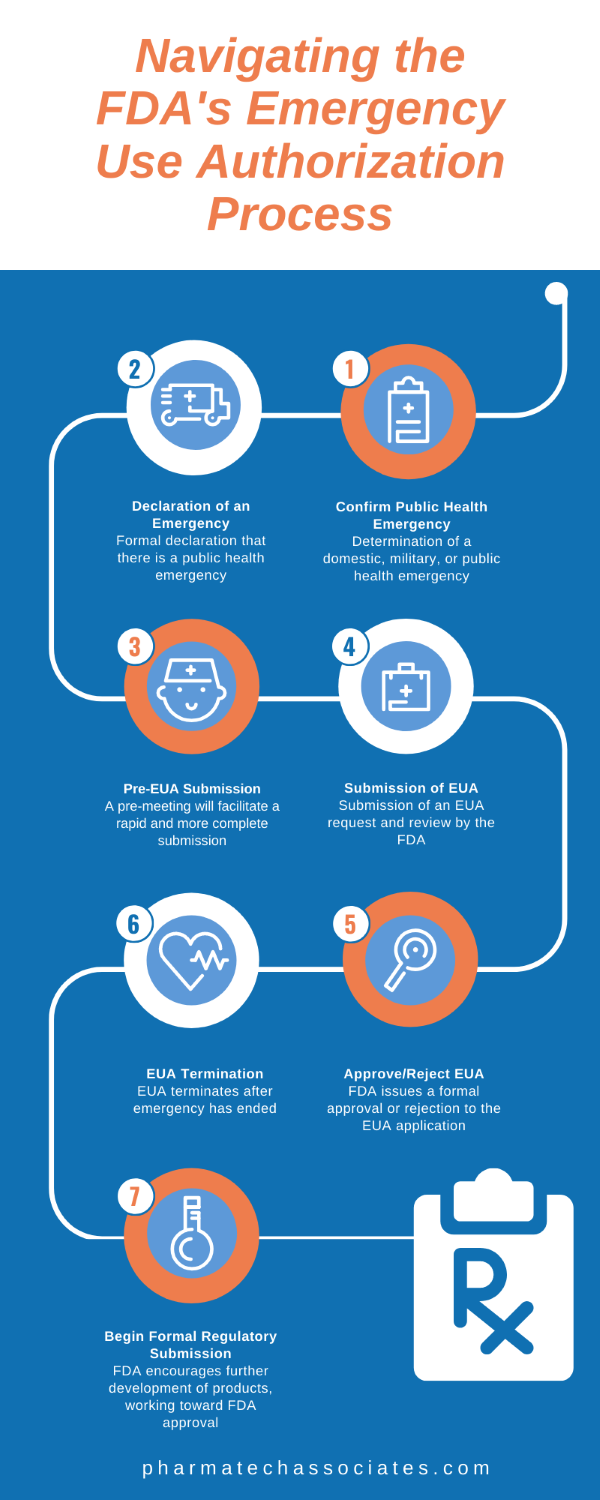
First, determination of a domestic, military, or public health emergency must be confirmed.
Once a formal declaration of a public health emergency has been made, the EUA Submission process can begin.
A pre-meeting will facilitate a rapid more complete EUA submission.
This involves submitting an EUA request to be reviewed by the FDA.
After reviewing the EUA application, the FDA issues a formal approval or rejection.
Once the public health emergency has ended, the EUA terminates.
The FDA encourages further development of products, working toward FDA approval.
Whether you are seeking FDA authorization, approval, or clearance, Pharmatech Associates can help you along the way. Contact us today!
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541