
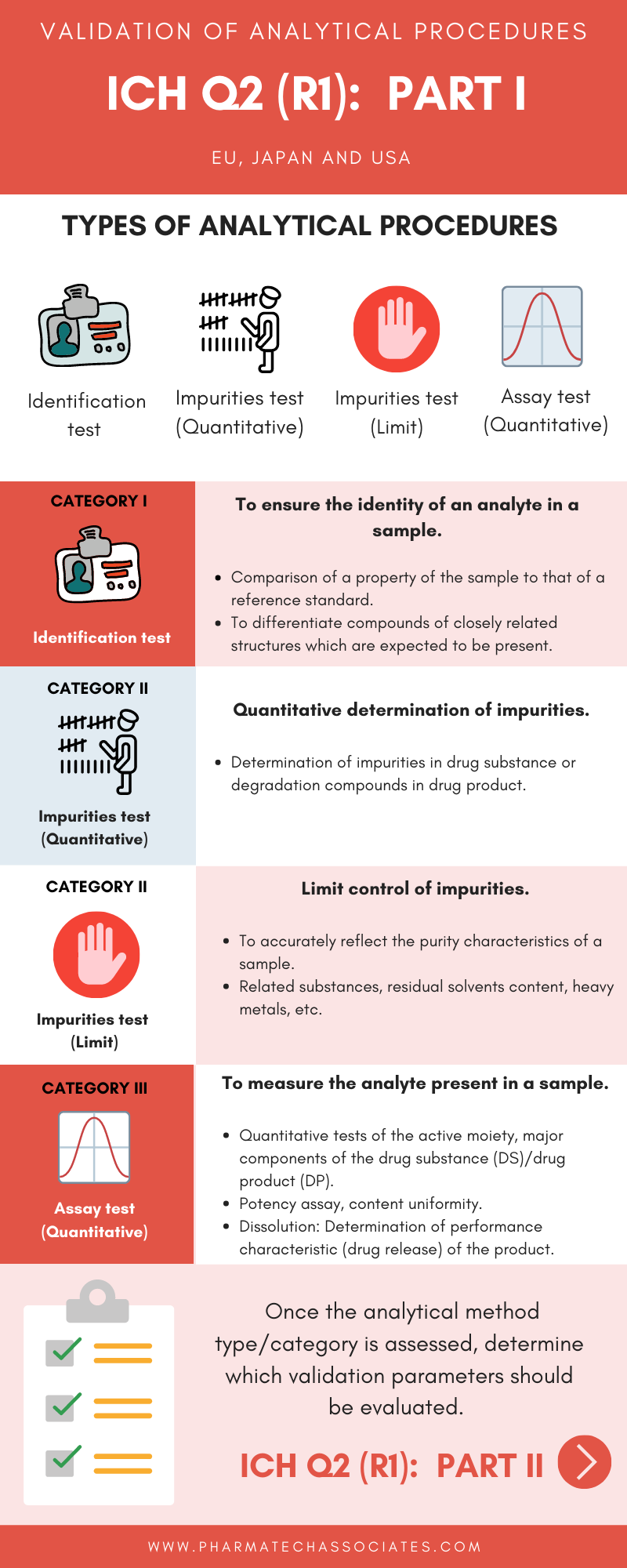
To ensure the identity of an analyte in a sample.
Quantitative determination of impurities.
To measure the analyte present in a sample.
Once the analytics method type/category is assessed, determine which validation parameters should be evaluated.
The experts at Pharmatech Associates can help you determine the analytical procedure that best fits your drug’s, biologic’s, or medical device’s development process. Contact us today!
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541