
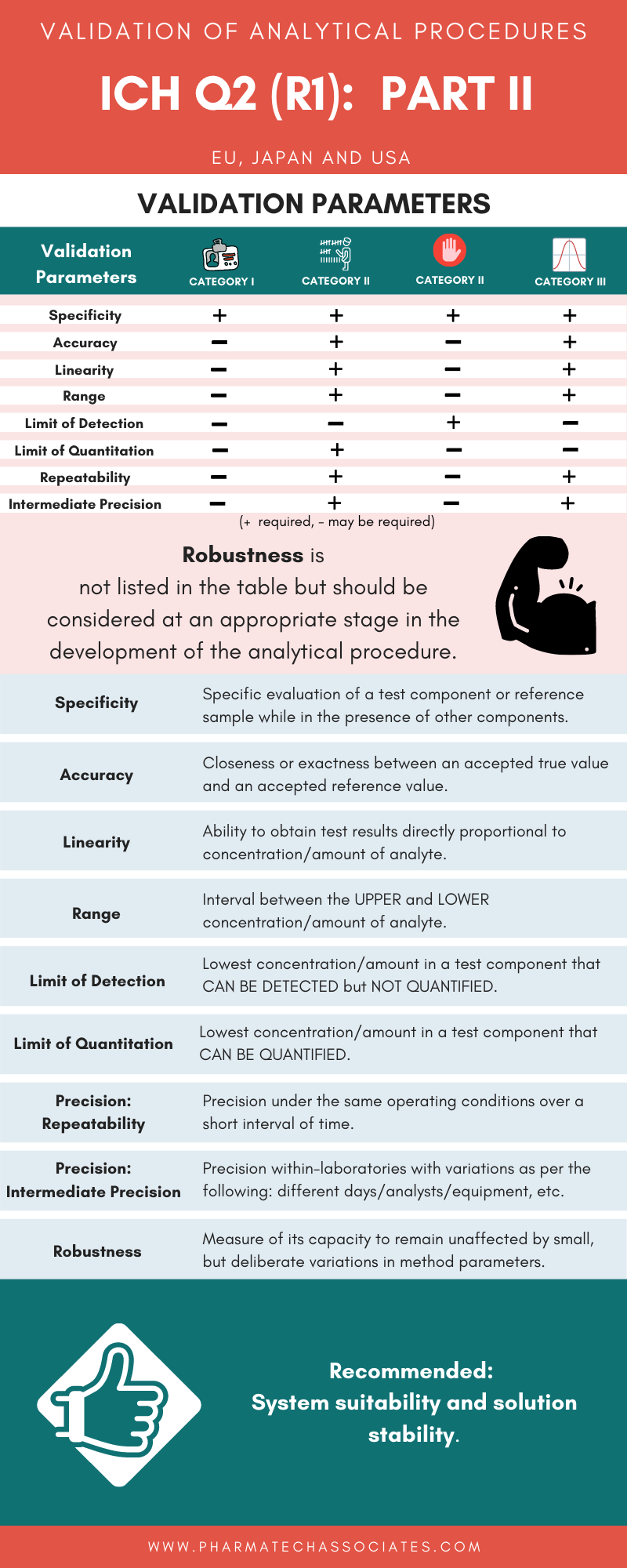
Specific evaluation of a test component or reference sample while in the presence of other components.
Closeness or exactness between an accepted true value and an accepted reference value.
Ability to obtain test results directly proportional to concentration/amount of analyte.
Interval between the UPPER and LOWER concentration/amount of analyte.
Lowest concentration/amount in a test component that CAN BE DETECTED but NOT QUANTIFIED.
Lowest concentration/amount in a test component that CAN BE QUANTIFIED.
Precision under the same operating conditions over a short interval of time.
Precision within-laboratories with variations as per the following: different days/analysts/equipment, etc.
Measure of its capacity to remain unaffected by small, but deliberate variations in method parameters.
Need help determining the validation parameters of your drug validation process? Pharmatech Associates can help! Contact us today.
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541