
You are not alone. Put simply, the Prescription Drug User Fee Act (PDUFA) mandates metrics for FDA to review and approve/reject marketing applications within 10 months.
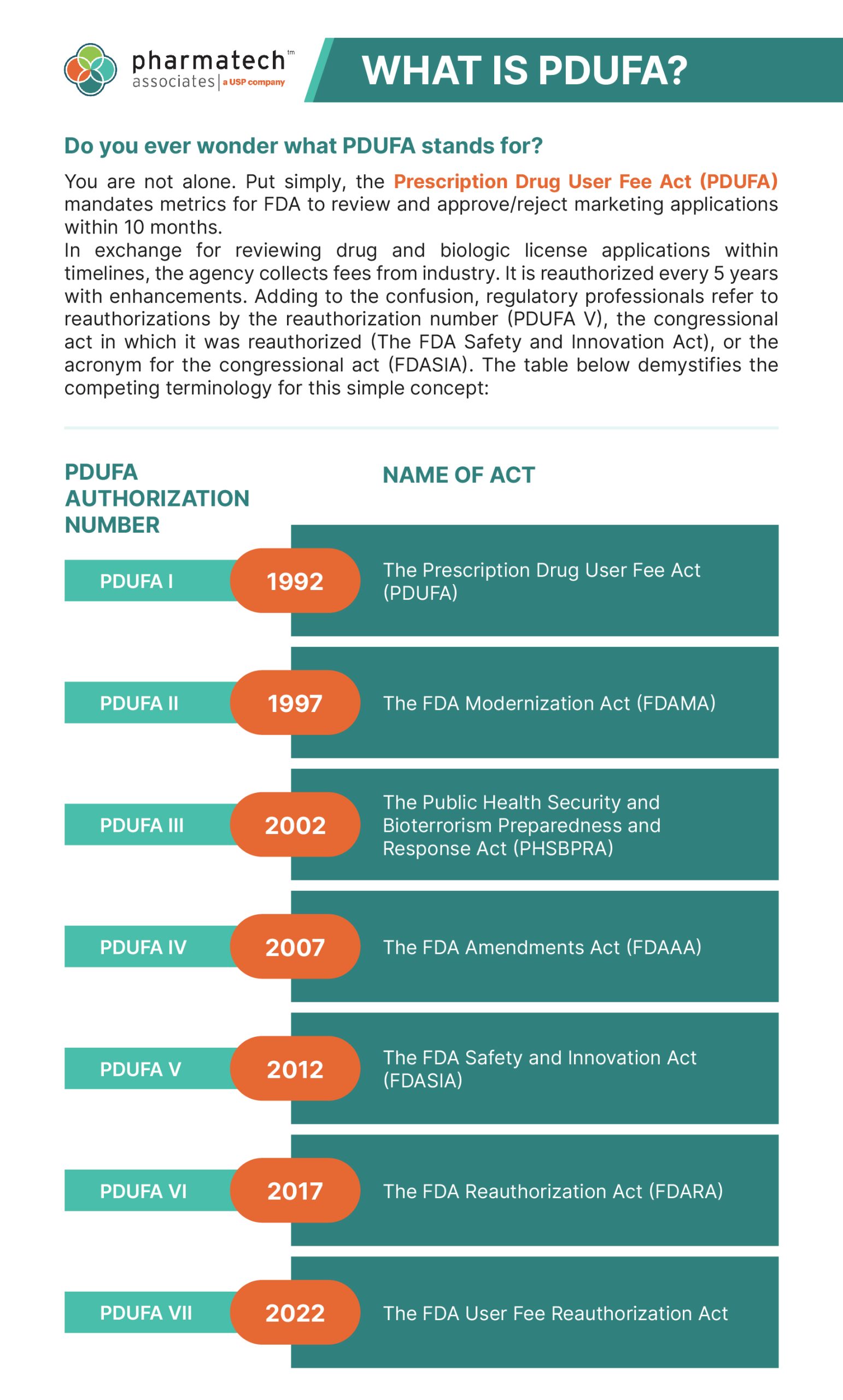
In exchange for reviewing drug and biologic license applications within timelines, the agency collects fees from industry. It is reauthorized every 5 years with enhancements. Adding to the confusion, regulatory professionals refer to reauthorizations by the reauthorization number (PDUFA V), the congressional act in which it was reauthorized (The FDA Safety and Innovation Act), or the acronym for the congressional act (FDASIA). The table below demystifies the competing terminology for this simple concept:
| PDUFA Authorization Number | Year | Name Of Act |
| PDUFA I | 1992 | The Prescription Drug User Fee Act (PDUFA) |
| PDUFA II | 1997 | The FDA Modernization Act (FDAMA) |
| PDUFA III | 2002 | The Public Health Security and Bioterrorism Preparedness and Response Act (PHSBPRA) |
| PDUFA IV | 2007 | The FDA Amendments Act (FDAAA) |
| PDUFA V | 2012 | The FDA Safety and Innovation Act (FDASIA) |
| PDUFA VI | 2017 | The FDA Reauthorization Act (FDARA) |
| PDUFA VII | 2022 | The FDA User Fee Reauthorization Act |
Our unique approach can help your company navigate the complexities of launching a drug, biologic or medical device into multiple markets. The first step? Connecting. Simply fill out our form and a representative will follow-up shortly.
1.877.787.0177
510.732.0177
22320 Foothill Blvd. Suite 330, Hayward CA 94541